*This post is also on the Bio in Wisconsin blog.*
One of the first things I learned about Srini Kalluri, founder, president, and CEO of Forte Research Systems, was that the ring tone on his phone is New York, New York. For those of you not familiar with the lyrics, the song includes this classic verse:
If I can make it there
I’ll make it anywhere
It’s up to you
New York, New York
The story was relayed as an explanation for the beginnings of OnCore, Forte’s Clinical Trial Management System (CTMS), which was rolled out first in National Cancer Institute (NCI) designated cancer centers. These centers are highly regulated with significant reporting and operational requirements, setting a high bar for a platform designed to manage a research organization.
Of the 45 OnCore customers, 25 are NCI-designated cancer centers, over 40% of such cancer centers that provide patient care. OnCore eClinical provides the electronic infrastructure to handle clinical research, including patient recruitment and management, investigator management, protocol tracking and adverse event reporting. The system is designed to interface with other software solutions that include billing, institutional review board review, grant and contact tracking, and electronic health records (EHR). An interesting feature of OnCore is that despite the complexity, the product is not customized for different organizations. Data exchange standards, such as the format and structure of the data, have been developed for various health related data. Health Level Seven International is one of the organizations that provide healthcare standards. OnCore has been developed to hand off data using data exchange standards, which allows a feature developed for one customer to be applied by other customers, even when their other software packages are different.
Forte Research (originally called PercipEnz Technologies) was founded in 2000 in Madison and has achieved the majority of its growth organically. The company started with friends and founders. In the lifetime of the company, just $1.8 million in investment capital has been raised, including an equity financing round with Wisconsin angels in 2007. In addition to OnCore, Forte has developed Allegro, a software as a service (SaaS) based trial management system for single or multi-site trials. Forte has 70 employees in the United States and plans to add half a dozen more in 2013. While development is done in the US, Forte started an operation in India two years ago that now has 20 employees. To provide some context for the funding of other clinical trial related software tools, consider San Francisco-based goBalto. goBalto raised a $12 million Series B round in 2012 for a SaaS-based clinical trial startup system designed for trial sponsors and contract research organizations to track trial data.
The company’s growth comes in part from the expansion of target customers from cancer centers to Clinical and Translational Science Award (CTSA) research institutions. In 2009, OnCore was enhanced to support multi-departmental research organizations such as Academic Medical Centers and CTSAs (Version 9.0). The National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS) launched the CTSA program in 2006 with the goal of improving translational science. The program identifies academic research institutions that collaborate on best practices in clinical research, training researchers and recruiting patients. The CTSA program now includes 61 medical research institutions across the country, 10 of which use OnCore to administer their clinical research portfolios.
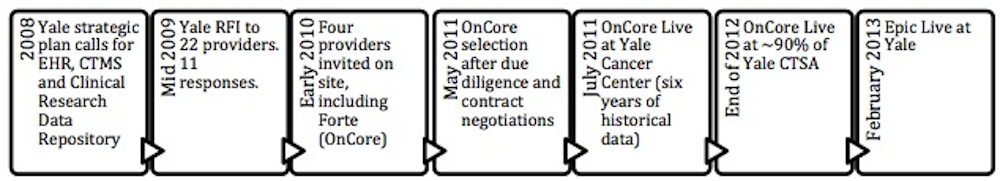
At their recent user group meeting co-hosted by Vanderbilt – Ingram Cancer Center in Nashville, there was a great case study for the overlapping roll out of OnCore as well as an electronic health record system at Yale University. The majority of the conference content is developed and presented by the research community. The Spring meeting had over 220 attendees, primarily OnCore users, representing 34 member institutions. Tesheia Johnson, Associate Director for Clinical Research at Yale School of Medicine and COO of Yale Center for Clinical Investigation (YCCI), talked about their very recent experience going live with two new systems: OnCore and the electronic health record, Epic.
YCCI provides infrastructure for clinical research, including training, for Yale School of Medicine and the Yale –New Haven hospital. While Yale’s YCCI started before the NIH initiative, Yale School of Medicine is now one of the sixty one CTSA programs across the country. Over the last five years, Yale moved forward to establish both an EHR and CTMS system, with OnCore and Epic both live by February 2013. The contract included a variety of desired features for Forte to build into OnCore, including customized reports, SAS data extracts, randomization functionality and automated clinical interfaces. In addition, Yale required significant integration with Epic, which had been selected as the EHR, to allow for features such as single data entry. These features have been implemented, which as mentioned above means they are now available to all customers with the OnCore eClinical system. Yale developed a website for users to learn about the OnCore tools. Epic went live at Yale in February 2013. One of the few features still being worked out is the interface with Beacon, the Epic oncology application, which is deeply integrated with clinical care.
 With both systems live, Johnson has already shifted focus to what Yale wants next from OnCore and Epic, which included increasing the interoperability of the two systems. The message that resonated strongly in the group was Johnson’s call for research institutions to collaborate, using their influence as customers to help guide the evolution of these critical software systems. She used a map that nicely highlighted that more than half of the CTSAs use the Epic EHR. As she had just listed Yale’s wish list for future enhancements to the OnCore eClinical system, Johnson also pointed out that research institutions should be strategic in participating in the Epic Research Advisory Councils and Forums, which allow users to share ideas AND influence what Epic should work on.
With both systems live, Johnson has already shifted focus to what Yale wants next from OnCore and Epic, which included increasing the interoperability of the two systems. The message that resonated strongly in the group was Johnson’s call for research institutions to collaborate, using their influence as customers to help guide the evolution of these critical software systems. She used a map that nicely highlighted that more than half of the CTSAs use the Epic EHR. As she had just listed Yale’s wish list for future enhancements to the OnCore eClinical system, Johnson also pointed out that research institutions should be strategic in participating in the Epic Research Advisory Councils and Forums, which allow users to share ideas AND influence what Epic should work on.
The next Onsemble conference will be co-hosted by the YCCI in New Haven. In addition to providing a forum for users to ask for new features, Forte is opening the Onsemble conference to all academic medical centers, regardless of whether they are OnCore users. With a push to a more active customer base, I suspect current users will recruit their colleagues to help drive the next generation of improvements in the clinical utility of these systems.


Be the first to comment